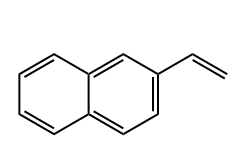
2-Vinylnaphthalene
2-Vinylnaphthalene
Factory sells 2-Vinylnaphthalene 827-54-3 with sufficient production capacity
- Molecular Formula:C12H10
- Molecular Weight:154.211
- Appearance/Colour:Tan powder
- Melting Point:64-68 °C(lit.)
- Boiling Point:270.9 °C at 760 mmHg
- Flash Point:115.5 °C
- PSA:0.00000
- Density:1.031 g/cm3
- LogP:3.48280
2-Vinylnaphthalene(Cas 827-54-3) Usage
|
General Description |
2-Vinylnaphthalene, also known as 2-vinyl-1-naphthalene, is a chemical compound with the formula C12H10. It is a white powder with a sweet, floral odor; 2-Vinylnaphthalene is an important organic compound with a wide range of applications in multiple fields: Synthesis of high-performance polymers: It serves as a monomer for the synthesis of various polymers with excellent properties. For example, by copolymerizing with other monomers, copolymers with special properties can be prepared, and these polymers are widely used in the fields of plastics, rubber, etc. Through polymerization with suitable monomers, materials with good mechanical properties, thermal stability, and chemical corrosion resistance can be obtained, which can be used for manufacturing auto parts, electronic device casings, and so on. |
InChI:InChI=1/C12H10/c1-2-10-7-8-11-5-3-4-6-12(11)9-10/h2-9H,1H2
827-54-3 Relevant articles
Photoredox Catalyzed Sulfonylation of Multisubstituted Allenes with Ru(bpy)3Cl2 or Rhodamine B
Chen, Jingyun,Chen, Shufang,Jiang, Jun,Lu, Qianqian,Shi, Liyang,Xu, Zekun,Yimei, Zhao
supporting information, (2021/11/09)
A highly regio- and stereoselective sulf...
Functionalized styrene synthesis via palladium-catalyzed C[sbnd]C cleavage of aryl ketones
Dai, Hui-Xiong,Wang, Xing,Wang, Zhen-Yu,Xu, Hui,Zhang, Xu
supporting information, (2022/03/31)
We report herein the synthesis of functi...
Palladium-Catalyzed Benzylic Silylation of Diarylmethyl Carbonates with Silylboranes under Base-Free Conditions
Asai, Kento,Hirano, Koji,Miura, Masahiro
supporting information, (2022/02/19)
A palladium-catalyzed benzylic silylatio...
Copper-Catalyzed Sulfonylation of Cyclobutanone Oxime Esters with Sulfonyl Hydrazides
Dong, Bingbing,Lu, Jiansha,Bao, Honghao,Zhang, Yuanyuan,Liu, Yingguo,Leng, Yuting
supporting information, p. 3769 - 3776 (2021/07/14)
A copper-catalyzed radical cross-couplin...
827-54-3 Process route
-
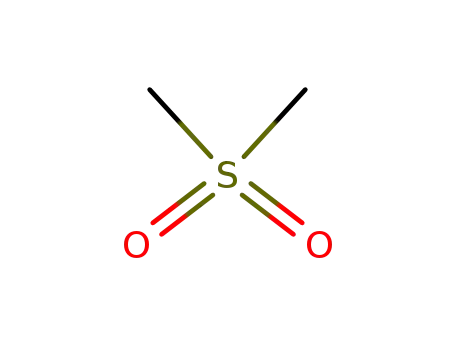
- 67-71-0
dimethylsulfone

-
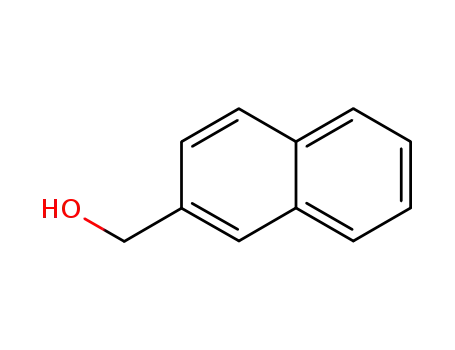
- 1592-38-7
2-Naphthalenemethanol

-
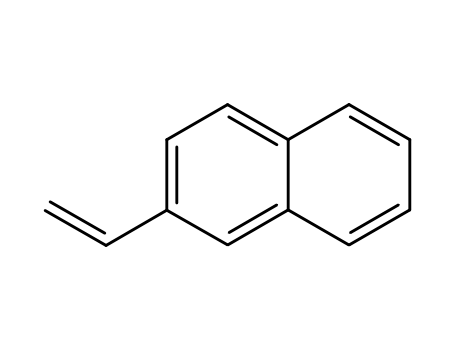
- 827-54-3,28406-56-6
2-naphthylethylene

-
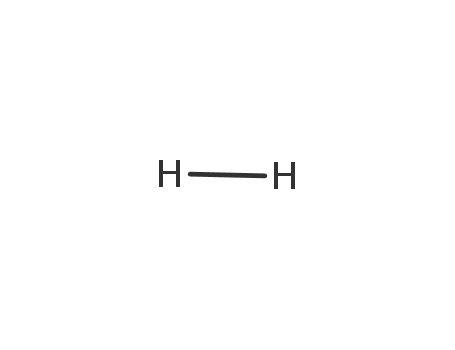
- 1333-74-0
hydrogen
| Conditions | Yield |
|---|---|
|
With 1,10-Phenanthroline; potassium tert-butylate; iron(II) chloride; In toluene; at 120 ℃; for 24h; Inert atmosphere; Schlenk technique;
|
83% |
-
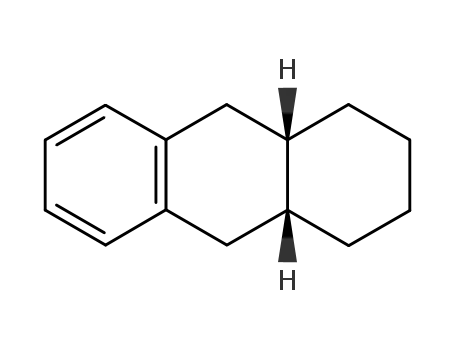
- 64363-88-8
cis-1,2,3,4,4a,9,10,10a-octahydrophenanthrene

-
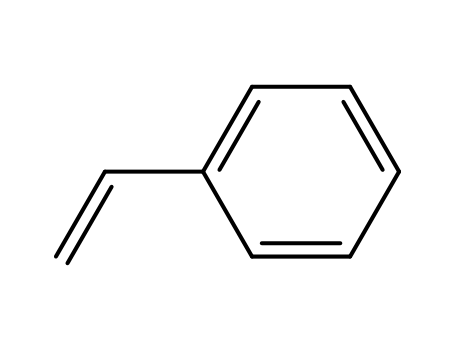
- 100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-
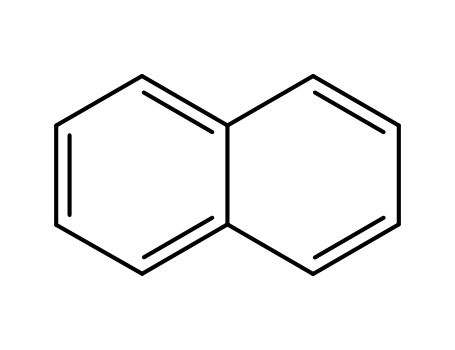
- 91-20-3,71998-51-1,72931-45-4
naphthalene

-
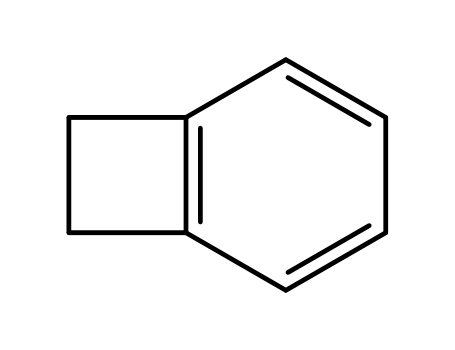
- 694-87-1
benzocyclobutene

-
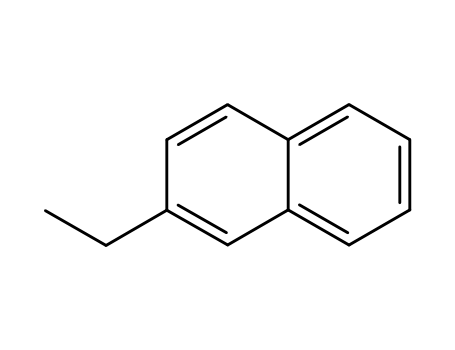
- 939-27-5
2-ethylnaphthalene

-

- 827-54-3,28406-56-6
2-naphthylethylene

-
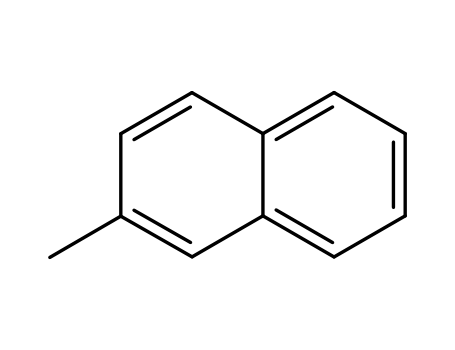
- 91-57-6,34468-07-0
2-Methylnaphthalene
| Conditions | Yield |
|---|---|
|
at 860 ℃; under 0.1 Torr; Product distribution; other temp.;
|
827-54-3 Upstream products
-
939-27-5

2-ethylnaphthalene
-
1485-07-0
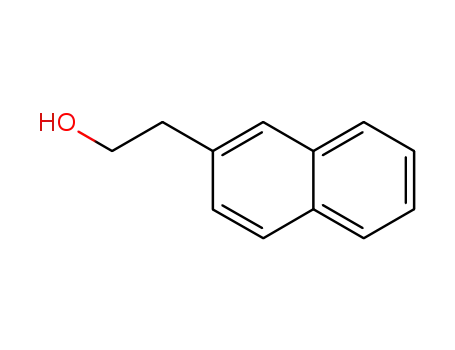
naphthalen-2-ethanol
-
32298-46-7
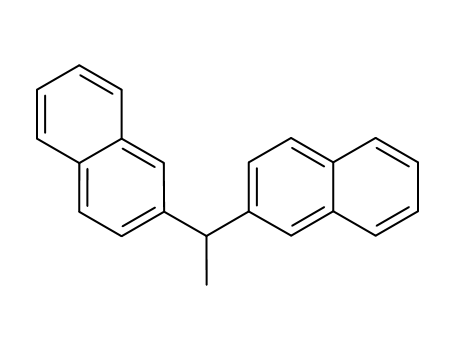
2,2'-(ethane-1,1-diyl)dinaphthalene
-
22364-54-1
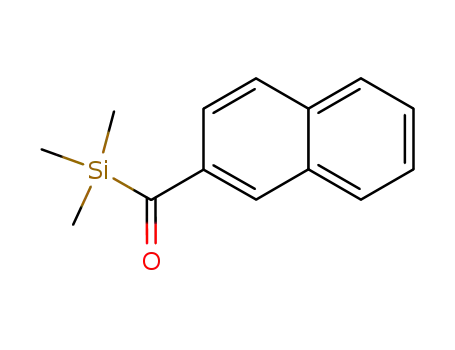
naphthalen-2-yl(trimethylsilyl)methanone
827-54-3 Downstream products
-
143879-48-5
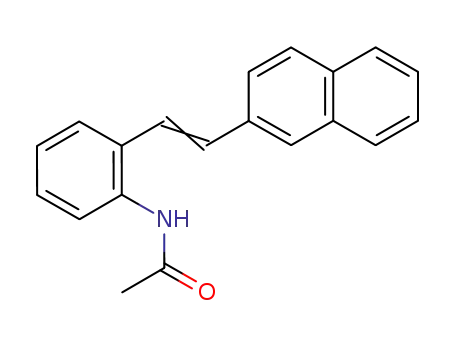
2-<2-(2-naphthyl)vinyl>acetanilide
-
939-27-5

2-ethylnaphthalene
-
136415-67-3
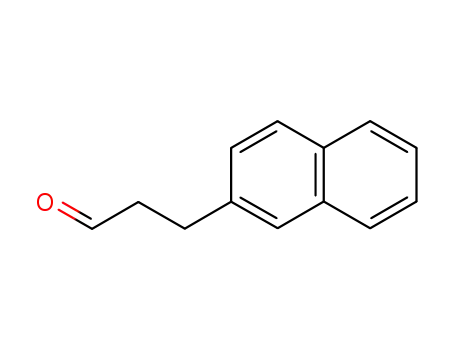
3-(naphthalen-2-yl)propanal
-
159759-69-0
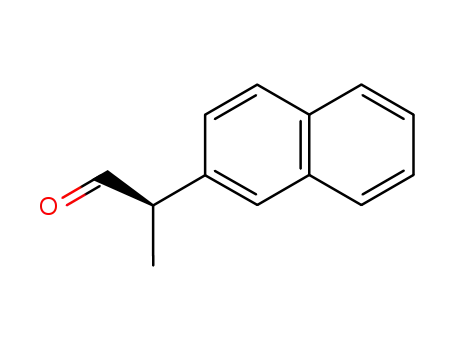
(R)-2-(naphthalen-2-yl)propanal
 2254784343
2254784343