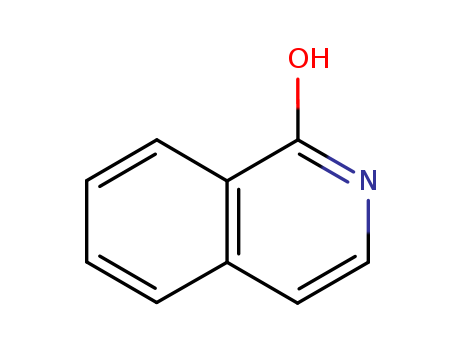
Isocarbostyril
Isocarbostyril
Factory Export Top Purity Isocarbostyril 491-30-5 In Stock
- Molecular Formula:C9H7NO
- Molecular Weight:145.161
- Appearance/Colour:Yellow solid
- Melting Point:212-216 °C
- Boiling Point:376.252 °C at 760 mmHg
- PKA:13.62±0.20(Predicted)
- Flash Point:218.788 °C
- PSA:33.12000
- Density:1.189 g/cm3
- LogP:1.94040
Isocarbostyril(Cas 491-30-5) Usage
|
Synthesis Reference(s) |
Journal of Heterocyclic Chemistry, 27, p. 1419, 1990 DOI: 10.1002/jhet.5570270544The Journal of Organic Chemistry, 21, p. 1337, 1956 DOI: 10.1021/jo01118a001Synthesis, p. 791, 1983 DOI: 10.1055/s-1983-30513 |
InChI:InChI=1/C9H7NO/c11-9-8-4-2-1-3-7(8)5-6-10-9/h1-6H,(H,10,11)
491-30-5 Relevant articles
-
Brown,White
, p. 1589,1591 (1957)
-
Carbon-13 and Proton NMR Spectra of 1(2H)-Isoquinolinone, 1(2H)-Phthalazinone, 4(3H)-Quinazolinone and their Substituted Derivatives
Spassov, S. L.,Atanassova, I. A.,Haimova, M. A.
, p. 795 - 799 (1985)
The 13C NMR chemical shifts, one-bond an...
-
Ono,Hata
, p. 3658,3660 (1973)
-
-
Hata
, p. 547 (1976)
-
Efficient visible light mediated synthesis of quinolin-2(1H)-ones from quinolineN-oxides
Bhuyan, Samuzal,Chhetri, Karan,Hossain, Jagir,Jana, Saibal,Mandal, Susanta,Roy, Biswajit Gopal
supporting information, p. 5049 - 5055 (2021/07/29)
Quinolin-2(1H)-ones are one of the impor...
Synthesis of Isoquinolones by Sequential Suzuki Coupling of 2-Halobenzonitriles with Vinyl Boronate Followed by Cyclization
Jaime-Figueroa, Saul,Bond, Michael J.,Vergara, J. Ignacio,Swartzel, Jake C.,Crews, Craig M.
, p. 8479 - 8488 (2021/06/28)
A novel, facile, and expeditious two-ste...
Ruthenium(II)-Catalyzed C?H Activation/Annulation of Aromatic Hydroxamic Acid Esters with Enamides Leading to Aminal Motifs
Dana, Suman,Sureshbabu, Popuri,Giri, Chandan Kumar,Baidya, Mahiuddin
supporting information, p. 1385 - 1389 (2021/02/26)
Hydroxamic acid ester directed C(sp2)?H ...
Synthesis of Overloaded Cyclopentadienyl Rhodium(III) Complexes via Cyclotetramerization of tert-Butylacetylene
Kolos, Andrey V.,Nelyubina, Yulia V.,Perekalin, Dmitry S.,Sundararaju, Basker
supporting information, p. 3712 - 3719 (2021/09/18)
Herein we describe the synthesis and rea...
491-30-5 Process route
-
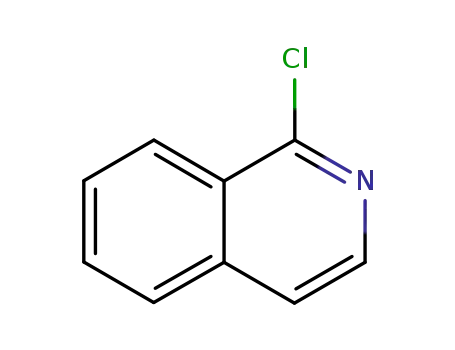
- 19493-44-8
1-chloroisoquinoline

-
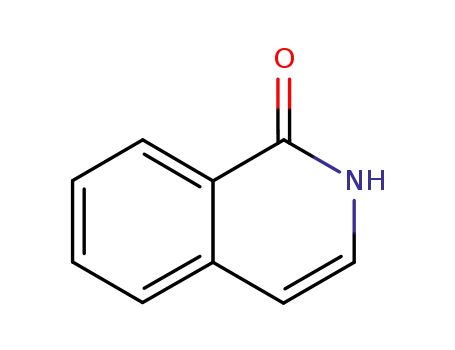
- 491-30-5
1-isoquinolone
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; In water; at 180 ℃; for 0.666667h; Microwave irradiation;
|
81% |
|
With ammonium acetate; acetic acid; at 100 ℃; for 3h;
|
80% |
|
With water; for 7h; Yield given; Heating;
|
|
|
Multi-step reaction with 3 steps
1: 24 percent / NaHSe / tetrahydrofuran; ethanol / 12 h / Ambient temperature
2: NaBH4 / ethanol / or in THF
3: 25 percent / tetrahydrofuran / or in EtOH
With sodium tetrahydroborate; sodium hydrogen selenide; In tetrahydrofuran; ethanol;
|
-
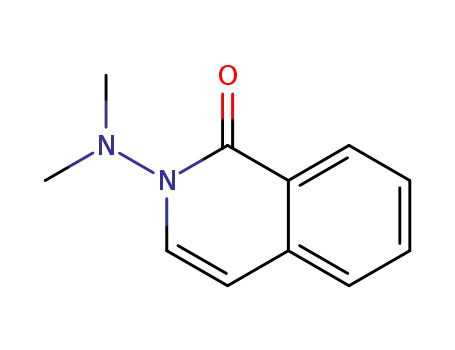
-
2-(dimethylamino)isoquinolin-1(2H)-one

-

- 491-30-5
1-isoquinolone
| Conditions | Yield |
|---|---|
|
With magnesium bis(monoperoxyphthalate)hexahydrate; In methanol; at 20 ℃; for 2h; Inert atmosphere;
|
68% |
491-30-5 Upstream products
-
67-56-1
methanol
-
7742-74-7
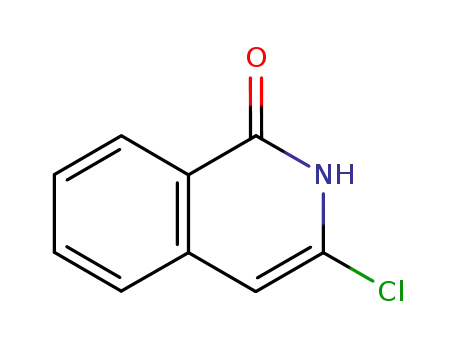
3-chloroisoquinolin-1(2H)-one
-
24623-16-3
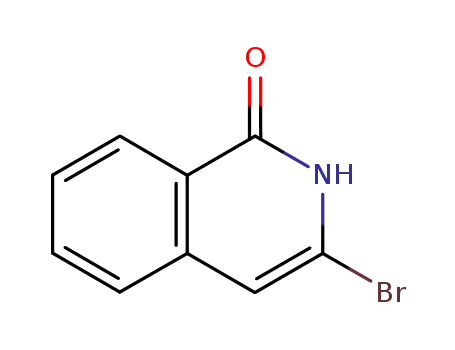
3-bromo-2H-isoquinolin-1-one
-
1532-72-5
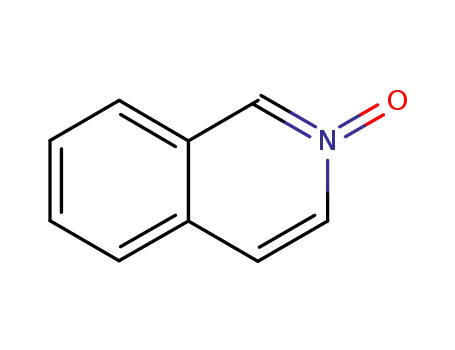
isoquinoline N-oxide
491-30-5 Downstream products
-
15793-94-9
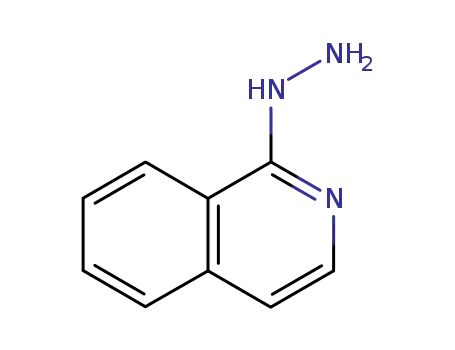
1-hydrazino-isoquinoline
-
119-65-3
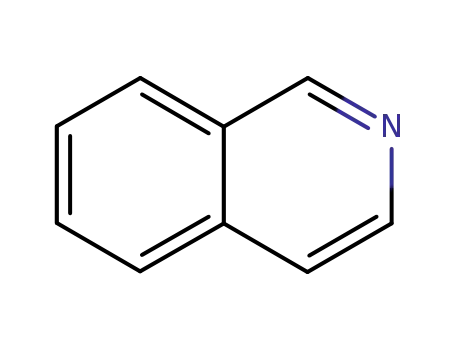
isoquinoline
-
19493-44-8

1-chloroisoquinoline
-
51206-40-7
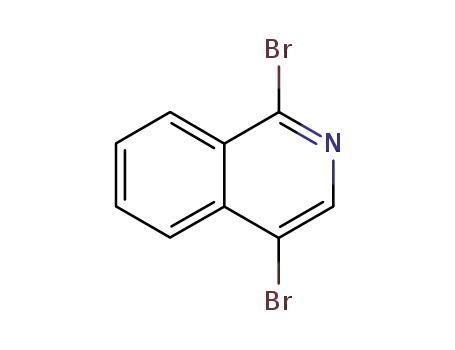
1,4-dibromoisoquinoline